Dive into the fascinating world of mole-number relationships, where chemistry’s quantitative backbone is revealed through intriguing questions. Whether you’re a student brushing up for exams or a science enthusiast, this quiz will sharpen your understanding of how moles connect to atoms, molecules, and more. Let’s see how well you can navigate through these essential chemical concepts!
We recommend that you do not leave the page that you are taking this quiz in. Stay honest 🙂
Mole-Number Relationships Quiz Questions Overview
1. What is Avogadro’s number?
6.022 x 10^23
1.602 x 10^-19
3.00 x 10^8
9.81 m/s^2
2. How many moles are in 12 grams of carbon-12?
1 mole
0.5 moles
2 moles
6.022 x 10^23 moles
3. Which of the following represents the number of molecules in 2 moles of water (H2O)?
1.204 x 10^24 molecules
6.022 x 10^23 molecules
3.011 x 10^23 molecules
2.408 x 10^24 molecules
4. What is the molar mass of sodium chloride (NaCl)?
58.44 g/mol
22.99 g/mol
35.45 g/mol
60.00 g/mol
5. How many atoms are in 3 moles of helium (He)?
1.806 x 10^24 atoms
6.022 x 10^23 atoms
3.011 x 10^23 atoms
9.033 x 10^24 atoms
6. If you have 1 mole of glucose (C6H12O6), how many carbon atoms do you have?
6.022 x 10^23 atoms
3.613 x 10^24 atoms
1.204 x 10^24 atoms
6 atoms
7. What is the mass of 0.5 moles of water (H2O)?
9 grams
18 grams
36 grams
1 gram
8. How many moles are in 22 grams of carbon dioxide (CO2)?
0.5 moles
1 mole
2 moles
22 moles
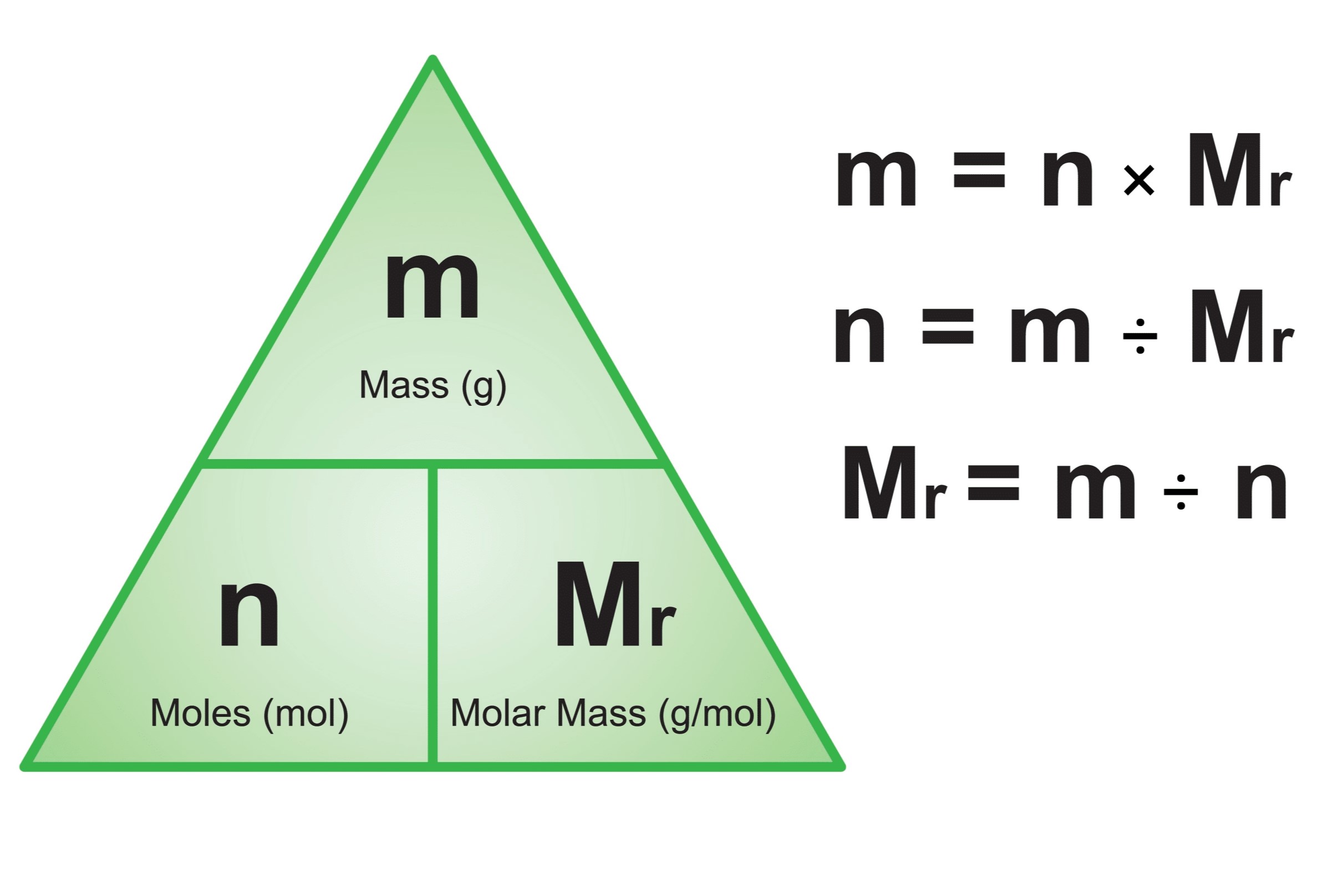
9. Which of the following is the correct formula for calculating the number of moles?
moles = mass / molar mass
moles = mass x molar mass
moles = molar mass / mass
moles = mass + molar mass
10. How many moles are in 16 grams of oxygen gas (O2)?
0.5 moles
1 mole
2 moles
4 moles
11. What is the volume of 1 mole of an ideal gas at standard temperature and pressure (STP)?
22.4 liters
1 liter
10 liters
44.8 liters
12. How many moles are in 98 grams of sulfuric acid (H2SO4)?
1 mole
0.5 moles
2 moles
98 moles
13. What is the mass of 2 moles of sodium (Na)?
45.98 grams
22.99 grams
11.50 grams
2 grams
14. How many moles are in 180 grams of glucose (C6H12O6)?
1 mole
0.5 moles
2 moles
180 moles
15. How many moles are in 50 grams of calcium carbonate (CaCO3)?
0.5 moles
1 mole
2 moles
50 moles
16. What is the molar mass of methane (CH4)?
16 g/mol
12 g/mol
4 g/mol
18 g/mol
17. How many moles are in 27 grams of aluminum (Al)?
1 mole
0.5 moles
2 moles
27 moles
18. How many moles are in 2 grams of hydrogen gas (H2)?
1 mole
0.5 moles
2 moles
4 moles
19. How many atoms are in 0.5 moles of iron (Fe)?
3.011 x 10^23 atoms
6.022 x 10^23 atoms
1.204 x 10^24 atoms
9.033 x 10^23 atoms
We recommend that you do not leave the page that you are taking this quiz in. Stay honest 🙂