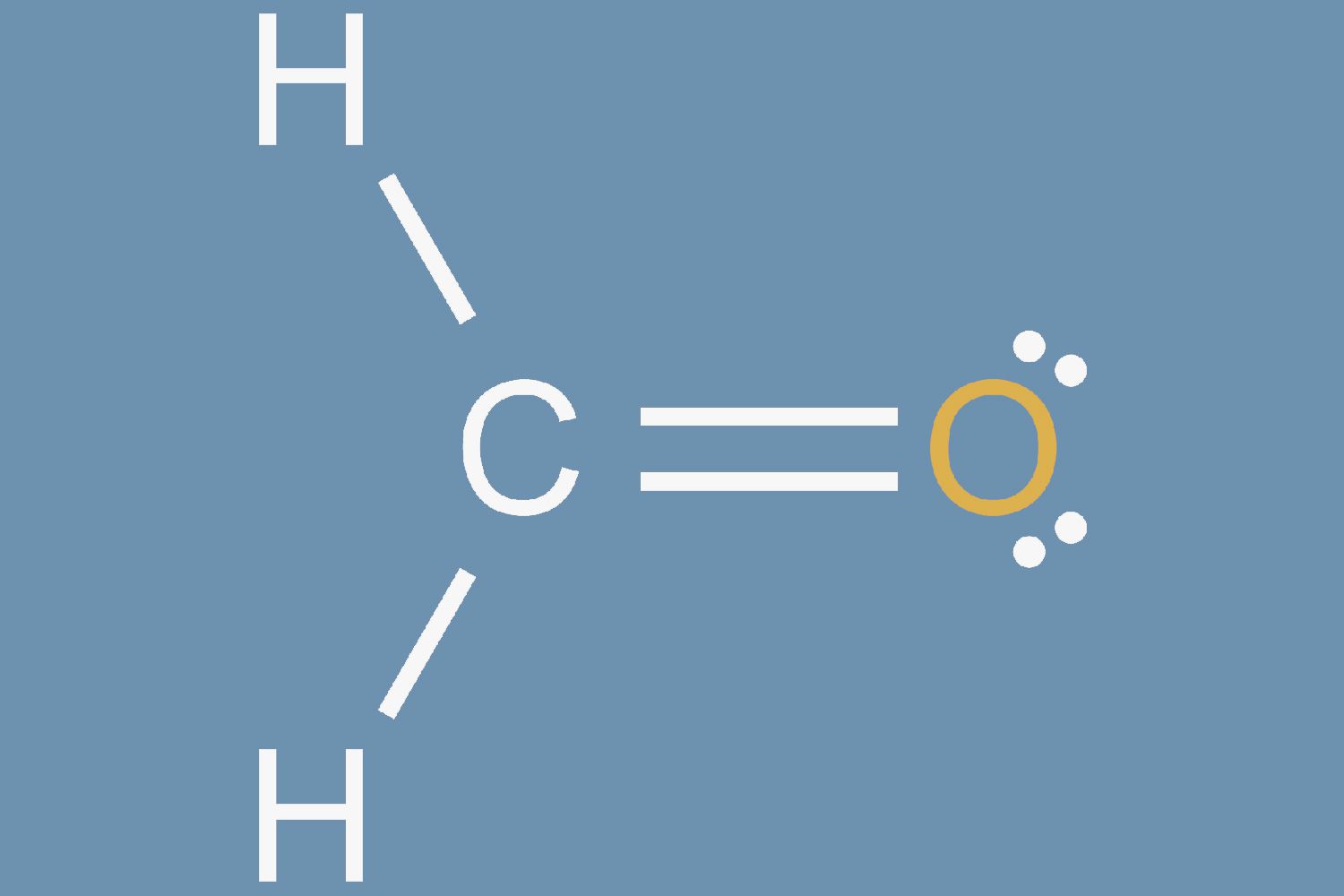
Dive into the intricate world of Lewis structures, where electrons dance around atoms to form bonds and create the molecules that make up our universe. This quiz will test your ability to visualize and draw these fundamental diagrams, essential for anyone studying chemistry. Sharpen your pencils and your minds, and get ready to master the art of Lewis structures!
We recommend that you do not leave the page that you are taking this quiz in. Stay honest 🙂
Lewis Structure Quiz Questions Overview
2. Which of the following molecules has a double bond in its Lewis structure?
O2
N2
H2
F2
3. How many lone pairs of electrons are on the central atom in the Lewis structure of ammonia (NH3)?
0
1
2
3
4. What is the molecular geometry of methane (CH4) according to its Lewis structure?
Linear
Trigonal planar
Tetrahedral
Bent
5. Which of the following molecules has a resonance structure?
CO2
H2O
O3
CH4
6. In the Lewis structure of carbon dioxide (CO2), how many double bonds are present?
0
1
2
3
7. What is the formal charge on the nitrogen atom in the Lewis structure of the nitrate ion (NO3-)?
0
+1
-1
+2
8. Which molecule has a trigonal planar geometry according to its Lewis structure?
BF3
NH3
H2O
CH4
9. How many lone pairs of electrons are on the central atom in the Lewis structure of sulfur hexafluoride (SF6)?
0
1
2
3
10. Which of the following ions has a Lewis structure with a single lone pair on the central atom?
NH4+
NO2-
CO3^2-
SO4^2-
11. What is the bond angle in the Lewis structure of water (H2O)?
90 degrees
104.5 degrees
120 degrees
180 degrees
12. Which molecule has a linear geometry according to its Lewis structure?
H2O
CO2
NH3
CH4
We recommend that you do not leave the page that you are taking this quiz in. Stay honest 🙂
Can Your Friends Do Better Than You in This Quiz?
Share this quiz with your friends and compare results.
Was this page helpful?
More Popular Chemistry Quizzes:
-
Stoichiometry Quiz
-
Periodic Table Symbols Quiz
-
Ionic Bonding Quiz
-
Strong Acids and Bases Quiz
-
Chemical Bonding Quiz
-
Basic Chemistry Quiz